Water quality
Explanations of water testing parameters:
The WATEX company conducts water chemical composition testing for the 6 most important water quality indicators that affect water quality (price 45.00 EUR without VAT).
We perform water analyzes for the following parameters:
- total water hardness;
- iron;
- ammonium ions;
- sulfate ions;
- oxidizability;
- electrical conductivity.
You will not only learn the chemical composition of water for 6 parameters, but also receive a free consultation and offer - a solution for your clean and tasty water. Water testing services we offer for people in the Baltic States.
WATEX water treatment solutions are recommended mainly based on the results of water analysis, so that the quality of the purified water meets the Cabinet of Ministers' regulations no. 671 on "Mandatory safety and quality requirements for drinking water".
Water quality indicators and their characteristics are described in more detail below:
 Color, turbidity - The turbidity and color of water depend mainly on elevated concentrations of iron, manganese, oxidizability (organics) and ammonium ions, as well as on environmental pollution (sand, clay, etc.). The color is accepted by consumers and without significant changes. In water supply systems, turbid water often occurs after plumbing repairs, disconnection or cleaning, etc. Turbid water can contain sand, mud, iron, organic matter and various deposits that clog the system, affect plumbing equipment, gauges and cause additional pressure losses.
Color, turbidity - The turbidity and color of water depend mainly on elevated concentrations of iron, manganese, oxidizability (organics) and ammonium ions, as well as on environmental pollution (sand, clay, etc.). The color is accepted by consumers and without significant changes. In water supply systems, turbid water often occurs after plumbing repairs, disconnection or cleaning, etc. Turbid water can contain sand, mud, iron, organic matter and various deposits that clog the system, affect plumbing equipment, gauges and cause additional pressure losses.
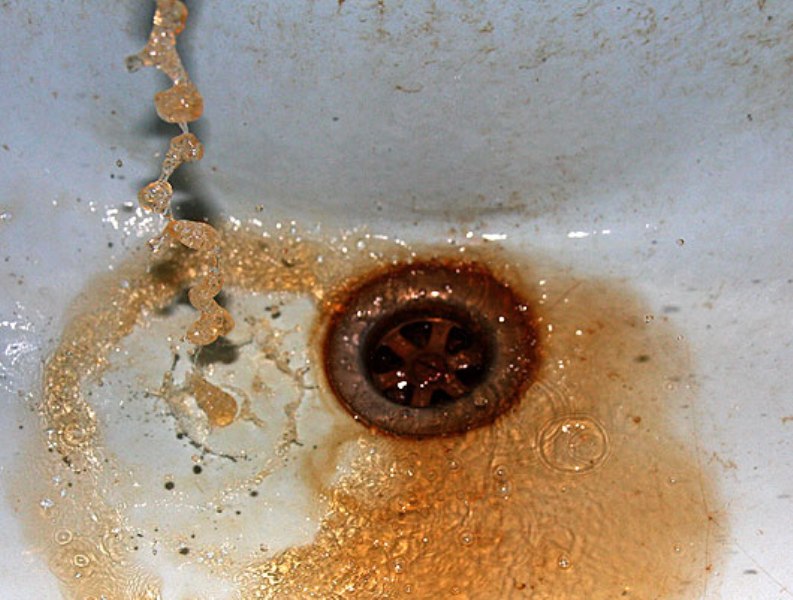 Iron (>0.2 mg / l) - Iron can be found in drinking water in three different ways. In dissolved form - water is clear, in precipitated form - water is cloudy, rust-colored and in colloidal form - water has a characteristic yellowish hue. Iron in drinking water promotes corrosion of pipelines and creates deposits in the pipelines, which increases the pressure losses in the pipelines and damages the plumbing equipment. Promotes the growth of iron bacteria, which over time can clog water systems and cause unpleasant odors.
Iron (>0.2 mg / l) - Iron can be found in drinking water in three different ways. In dissolved form - water is clear, in precipitated form - water is cloudy, rust-colored and in colloidal form - water has a characteristic yellowish hue. Iron in drinking water promotes corrosion of pipelines and creates deposits in the pipelines, which increases the pressure losses in the pipelines and damages the plumbing equipment. Promotes the growth of iron bacteria, which over time can clog water systems and cause unpleasant odors.
Manganese ions (>0.05 mg / l) - Elevated content of manganese ions in water causes brownish-red or black deposits on dishes and sanitary equipment, as well as metallic taste of water.
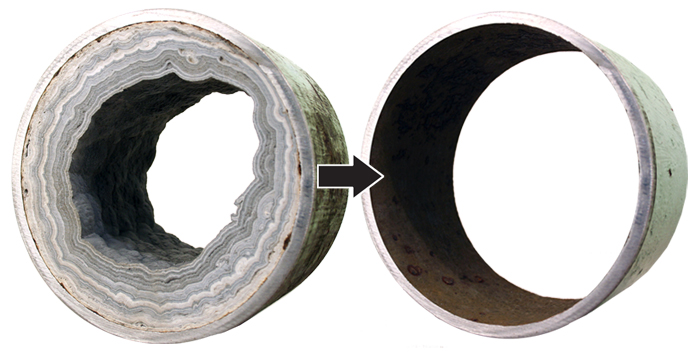 Total hardness - Total hardness is the amount of calcium and magnesium ions in the water. The maximum permissible limits of the total water hardness for drinking water are not specified by the local authorities. Water with a total hardness of up to 2.4 mg-eq / l is considered to be soft water, but higher than 2.4 mg-eq / l is considered hard water. Hard water creates deposits on various household appliances, heating element surfaces, pipelines thus damaging the equipment and increasing pressure losses in the pipelines. Also hard water can increase the use of cleaning and washing supplies and reduces energy effeciency.
Total hardness - Total hardness is the amount of calcium and magnesium ions in the water. The maximum permissible limits of the total water hardness for drinking water are not specified by the local authorities. Water with a total hardness of up to 2.4 mg-eq / l is considered to be soft water, but higher than 2.4 mg-eq / l is considered hard water. Hard water creates deposits on various household appliances, heating element surfaces, pipelines thus damaging the equipment and increasing pressure losses in the pipelines. Also hard water can increase the use of cleaning and washing supplies and reduces energy effeciency.
 Hydrogen sulphide - A colorless and volatile gas which gives the water an unpleasant smell of rotten eggs, but when it reacts with oxygen for a long time, the smell disappears. This smell mostly can be observed in boreholes, where the water is in an anaerobic environment (without oxygen). Due to the high pressure from the earth the hydrogen sulphide gas that accumulates undergound can get mixed up with water.
Hydrogen sulphide - A colorless and volatile gas which gives the water an unpleasant smell of rotten eggs, but when it reacts with oxygen for a long time, the smell disappears. This smell mostly can be observed in boreholes, where the water is in an anaerobic environment (without oxygen). Due to the high pressure from the earth the hydrogen sulphide gas that accumulates undergound can get mixed up with water.
 Mineralization - The sum of total dissolved salt ions. Waters with a mineralization of less than 1 g / l are considered as fresh waters, but those with a higher mineralization are considered as mineral waters. High levels of mineralization can significantly reduce the effectiveness of water disinfectants and promote corrosion of pipelines.
Mineralization - The sum of total dissolved salt ions. Waters with a mineralization of less than 1 g / l are considered as fresh waters, but those with a higher mineralization are considered as mineral waters. High levels of mineralization can significantly reduce the effectiveness of water disinfectants and promote corrosion of pipelines.
Alkalinity - An indicator of the resistance of water to a decrease in pH with the addition of acid. Low alkalinity increases pH sensitivity. Water with low alkalinity can quickly change from high pH to low pH and then return again. High total alkalinity reduces pH sensitivity.
Sodium ions (<200 mg / l) - Increased sodium ions increase water mineralization and promote corrosion of pipelines. Excessive amounts of sodium ions make water salty.
Chloride ions (<250 mg / l) - Excessive amounts of chloride ions make water salty, which can signal that in the borehole some water is seawater (seawater intrusion). Chloride ions, like sodium ions, increase the mineralization of water and the electrical conductivity of water, thus increasing the corrosivity of water.
Sulphate ions (<250 mg / l) - The high presence of sulphate ions in the water impairs the taste of the water, increases the electrical conductivity and mineralization of the water, promotes corrosion of the pipes and creates deposits on the walls of the pipes.
Hydrocarbon ions - Hydrocarbonates are the main alkalinity factor in water, so their properties are similar to alkalinity. Increased bicarbonate content affects the electrical conductivity of water and increases corrosion of metal pipes. Under the influence of high temperatures, calcium and magnesium bicarbonates cause deposits on the heating elements as well as form a particle layer inside the pipeline.
 pH - Water with a pH of 0 to 6.5 forms an acidic environment, a pH of 6.5 to 9.5 forms a neutral environment and is acceptable to consumers, a pH above 9.5 forms a basic environment. Low pH water promotes corrosion of pipes and other sanitary ware, while an alkaline environment creates sediment and reduces disinfection efficiency.
pH - Water with a pH of 0 to 6.5 forms an acidic environment, a pH of 6.5 to 9.5 forms a neutral environment and is acceptable to consumers, a pH above 9.5 forms a basic environment. Low pH water promotes corrosion of pipes and other sanitary ware, while an alkaline environment creates sediment and reduces disinfection efficiency.
Electrical conductivity (<2500 S / cm) - Increased electrical conductivity indicates high levels of dissolved salts, high hardness and mineralization in water. Electrochemical processes take place in water with high electrical conductivity, which promotes corrosion of pipelines and other equipment.
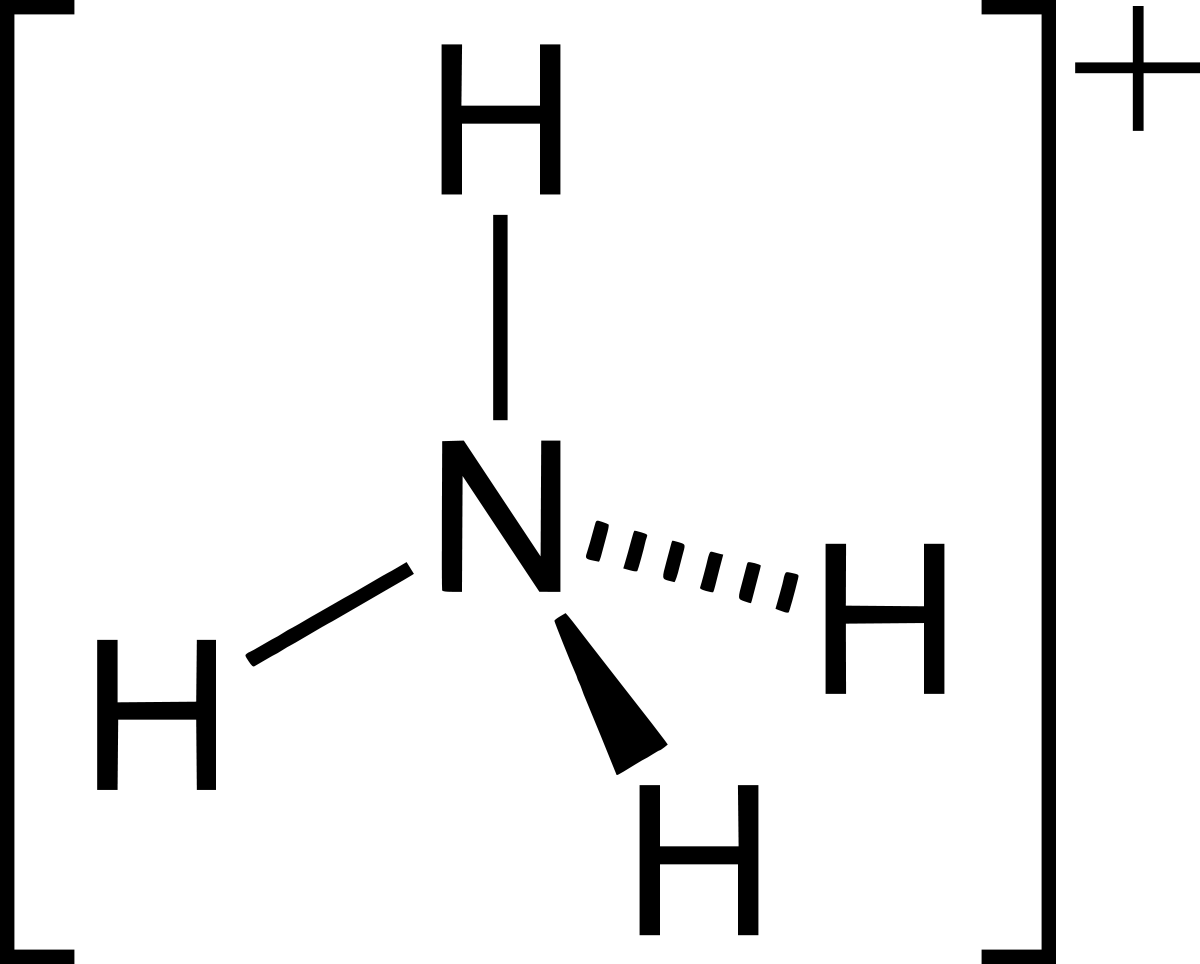 Ammonium ions (<0.5 mg / l) - Excessive concentrations of ammonium ions in water can damage the taste and smell of water and reduce its disinfection efficiency by reducing it. Promotes the growth of algae by creating deposits in pipelines and other sanitary equipment, as well as stains the water greenish. Elevated ammonium concentrations in drinking water may indicate environmental pollution at the source of the water source, the presence of wastewater or agricultural fertilizers in the water. The risk to human health from the use of water with a slightly high amount of ammonium ions is relatively negligible.
Ammonium ions (<0.5 mg / l) - Excessive concentrations of ammonium ions in water can damage the taste and smell of water and reduce its disinfection efficiency by reducing it. Promotes the growth of algae by creating deposits in pipelines and other sanitary equipment, as well as stains the water greenish. Elevated ammonium concentrations in drinking water may indicate environmental pollution at the source of the water source, the presence of wastewater or agricultural fertilizers in the water. The risk to human health from the use of water with a slightly high amount of ammonium ions is relatively negligible.
 Oxidizability (<5 mg O / l ) - Oxidizability refers to the amount of oxygen required to oxidize organic impurities in water. Increased oxidizability concentrations are observed in groundwater and surface water, where environmental pollutants have a large impact on water quality, less this indicator is observed in deep boreholes. High concentrations of oxidizability in water affect the color of the water, making the water yellowish or brownish. High oxidizability content indicates high contamination of organic matter in water. Organic substances in their pure form do not endanger human health, but they are harmful through interactions with iron and manganese, which adversely affect the human digestive and endocrine systems.
Oxidizability (<5 mg O / l ) - Oxidizability refers to the amount of oxygen required to oxidize organic impurities in water. Increased oxidizability concentrations are observed in groundwater and surface water, where environmental pollutants have a large impact on water quality, less this indicator is observed in deep boreholes. High concentrations of oxidizability in water affect the color of the water, making the water yellowish or brownish. High oxidizability content indicates high contamination of organic matter in water. Organic substances in their pure form do not endanger human health, but they are harmful through interactions with iron and manganese, which adversely affect the human digestive and endocrine systems.
Nitrate ions / nitrite ions (<50 mg / l / 0.5 mg / l) - Shows the effect of environmental pollution on the water source or the presence of sewage or fertilizers in the water. High levels of nitrite are toxic to humans and animals, especially infants. It can enter the body as nitrate, a nutrient that is essential for plant growth, and can be converted to nitrite, which disrupts the ability of hemoglobin to supply oxygen to the blood.
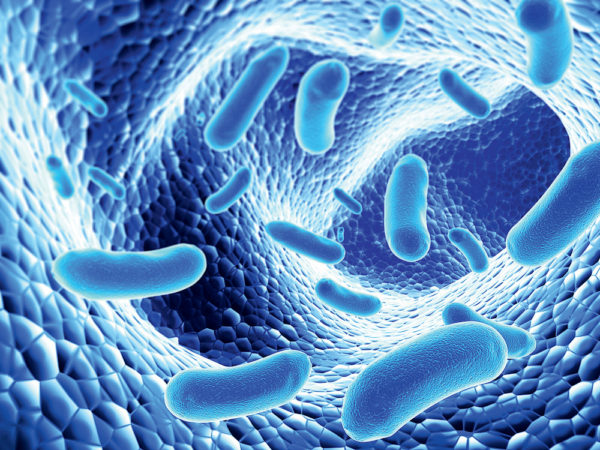 Viruses and bacteria - Pathogenic bacteria and other viruses are very dangerous to human health, but their presence in water cannot be assessed visually by looking at the water due to the small size of the microorganisms. Bacteriological water pollution is most common in surface waters (rivers, lakes, ponds, open wells).
Viruses and bacteria - Pathogenic bacteria and other viruses are very dangerous to human health, but their presence in water cannot be assessed visually by looking at the water due to the small size of the microorganisms. Bacteriological water pollution is most common in surface waters (rivers, lakes, ponds, open wells).
 +371 67381989
+371 67381989
